
Both vorozole and letrozole were initially developed and underwent clinical trials as antineoplastic agents. Vorozole and letrozole (Figure 1) are nonsteroidal, triazole-containing compounds that are competitive, reversible, third-generation aromatase (CYP19A1) inhibitors.

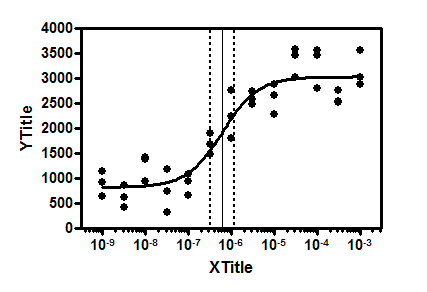
Since CYP3A4 makes up the majority of the CYP content found in the human liver, and vorozole inhibits it moderately well but letrozole does not, CYP3A4 is a good candidate for the protein that -vorozole is binding to in the liver. Letrozole is only a moderate inhibitor of CYP1A1 and CYP2A6 (IC 50 = 69.8 and 106 μM) and a very weak inhibitor of CYP3A4 (<10% inhibition at 1 mM). It was determined that vorozole is a potent inhibitor of CYP1A1 (IC 50 = 0.469 μM) and a moderate inhibitor of CYP2A6 and CYP3A4 (IC 50 = 24.4 and 98.1 μM, resp.). In search of finding the protein responsible for the accumulation of vorozole in the liver, fluorometric high-throughput screening assays were used to test the inhibitory capability of vorozole and letrozole on a series of liver cytochrome P450s (CYP1A1, CYP1A2, CYP2A6, and CYP3A4).
Pretreatment with letrozole does not affect the binding of vorozole in the liver. Vorozole can be used as a radiotracer for aromatase in living animals but when administered by IV, it collects in the liver. Vorozole and letrozole are third-generation aromatase (cytochrome P450 19A1) inhibitors.


 0 kommentar(er)
0 kommentar(er)
